Testing Services Offered by TestCert Lab
TestCert Lab specializes in offering advanced and compliant testing solutions across the following sectors:

Medical Sector
TestCert provides comprehensive testing services for medical devices in line with global regulatory expectations.

Leather & Textile
Our Colorfastness to Rubbing test evaluates how well dyed or printed textiles resist color transfer when subjected to dry and wet rubbing.

Packaging Sector
Our Drop Test service evaluates a package or product’s ability to withstand impacts and shocks during handling, transportation, and distribution.
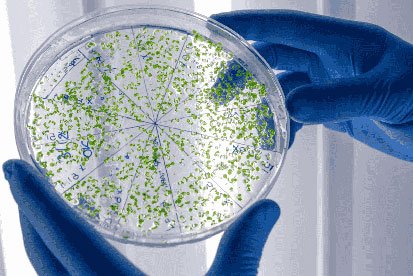
Environmental Monitoring
Maintaining a clean and compliant environment is crucial in healthcare, pharmaceuticals, food production,

Food
Pharmaceuticals Testing is a critical analytical procedure used to determine the rate and extent to which an active pharmaceutical ingredient.

Pharmaceuticals
Pharmaceuticals Testing is a critical analytical procedure used to determine the rate and extent to which an active pharmaceutical ingredient.
Our Scale and Reach to Client Satisfaction
Clients Testimonial

Dr. Ayesha Malik
Usman Tariq
Zeeshan Rauf
Our Latest Research
Our modern facility is equipped to handle a wide range of medical device evaluations, ensuring your products meet regulatory standards with confidence.
Service FAQ
Below are the frequently asked questions for placement at the bottom of the TestCert Laboratory website.
1. What services does TestCert Laboratory provide?
We provide multi-sector testing, validation, and certification support across Medical Devices, Packaging, Food, Environmental, Leather & Textile, Cosmetics and Pharmaceuticals.
2. Are your test results internationally accepted?
Yes. All results are ISO/IEC 17025:2017-compliant and recognized by global regulatory bodies and markets.
3. How do you ensure accuracy and reliability?
Through validated methods, calibrated instruments, advanced equipment, and strict Good Laboratory Practices and Good Hospital Practices.
4. Do you only provide in-house testing?
We specialize in in-house testing and partner with accredited labs for specialized parameters.
5. Can you customize test plans?
6. Which industries benefit from your services?
Medical, food, textile, leather, packaging, cosmetics, and environmental monitoring industries.
7. What standards and regulations do you follow?
We comply with ISO, ASTM, EN, BS, AOAC, EU (MDR) , FDA, Codex, and WHO guidelines.
8. What makes TestCert different?
Our multi-sector expertise, advanced methods, and client-centered approach ensure both compliance and market acceptance.
9. Do you provide training or consultancy?
Yes. We offer regulatory training, ISO workshops, validation consultancy, and documentation support.
10. How can I get started?
Contact us via website, email, or phone. Our team guides you from submission to reporting.
11. How many test samples are required?
Sample size depends on product type, complexity, risks, and applicable standards.
12. What if my product fails or partially complies?
We provide a detailed report, and clients can make corrections or retest before market launch.
